
Insight Magnetics is a small research and development business with 30 years of experience in sensitive magnetic measurements. Our mission today is to make reliable, noninvasive liver iron measurements available in any hospital or clinic, for the management of iron overload disease. This webpage describes why that mission is important and how we are working to accomplish it. The information is organized into the following topics:
The need for this technology.
Our solution.
Latest results.
Previous results.
How our system works.
Main components of our system.
How liver iron measurements are done.
Research collaborations.

The need. Millions of patients around the world are at risk for iron overload, an accumulation of too much iron in the body. That excess iron can come from the repeated blood transfusions that are needed in leukemia, myelodysplastic syndrome, and inherited blood disorders such as thalassemia and sickle-cell disease. It can come from genetic disorders such as hereditary hemochromatosis or African and African-American iron overload, which cause the body to absorb too much iron from food. It can also arise from alcoholic, nonalcoholic and viral liver disease. Depending on the disease, this excess iron can cause problems as varied as liver cirrhosis and liver cancer, diabetes and endocrine problems, infections, or congestive heart failure.
To manage iron overload, doctors need to know how much iron the patient has. However, the only ways of measuring excess iron today are blood tests, which are unreliable, liver biopsy, which is painful and dangerous, and magnetic resonance imaging (MRI), which may cost hundreds of dollars and take weeks or months to schedule.
What is needed is an iron-overload test that is affordable, reliable, non-invasive and available on demand, in any hospital or clinic. Our technology is designed to meet that need.
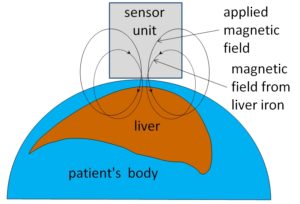
Our solution. Our technology uses a sensor unit next to the patient’s skin to measure the magnetic susceptibility of a patient’s liver, that is, how strongly it becomes magnetized when placed in a magnetic field. This magnetic susceptibility is proportional to the concentration of iron in the liver, which is known from earlier research to be a reliable indicator of the total amount of iron in the body.
This method does not break the skin or make electrical contact with the body. The only thing that enters the body is the magnetic field that we apply. That magnetic field is considerably weaker than the ones used in magnetic resonance imaging.
Previous devices using this magnetic sensing technique rely on sophisticated magnetic sensors called SQUIDs (Superconducting QUantum Interference Devices). These devices are expensive and complicated, and require liquid helium. Only four SQUID liver-iron sensors are in use today. In contrast, our device uses inexpensive magnetic sensors that work at room temperature. We call it a room-temperature susceptometer (RTS).
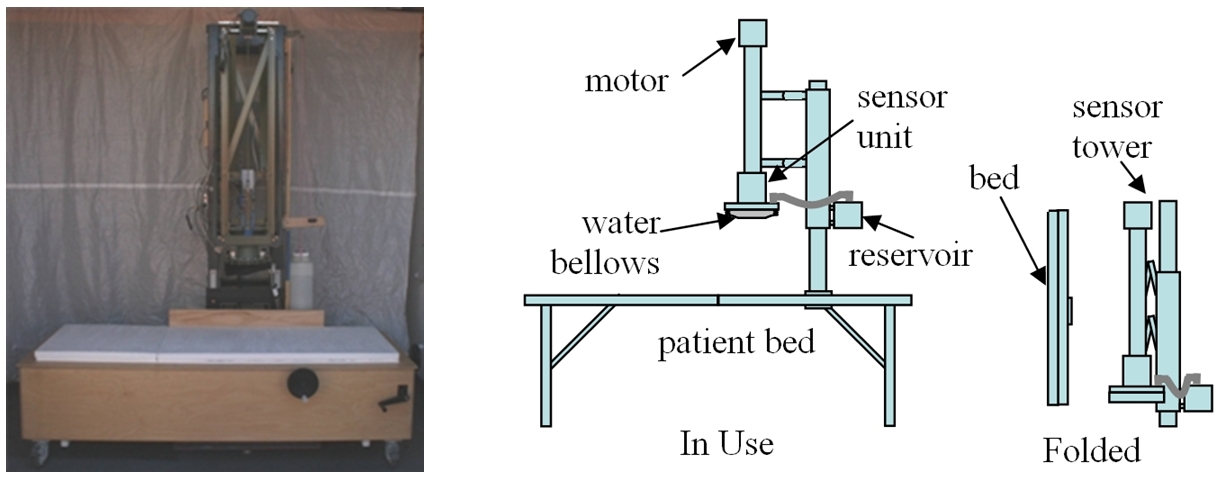
The RTS can be installed in any room, and requires no special infrastructure. Our existing device (above, left) is already much smaller and less expensive than the SQUID. A future version (above, right) will break down to fit into the trunk of a car.
 Latest results. The graph at left compares liver iron concentrations (LICs) from our RTS and the SQUID susceptometer at Benioff Children’s Hospital, Oakland, California. LIC values from the two susceptometers are in very good agreement, with a Pearson product moment correlation coefficient r of 0.986. This work is described in a recent abstract.
Latest results. The graph at left compares liver iron concentrations (LICs) from our RTS and the SQUID susceptometer at Benioff Children’s Hospital, Oakland, California. LIC values from the two susceptometers are in very good agreement, with a Pearson product moment correlation coefficient r of 0.986. This work is described in a recent abstract.
These results are consistent with the hypothesis that the RTS’s accuracy is equivalent to the SQUID’s, although the number of patients is too small to demonstrate this hypothesis with high statistical confidence. Our next goal is to test the RTS’s accuracy using the Oakland SQUID and MRI in a larger number of patients.
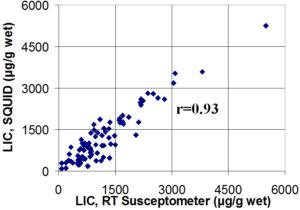
Previous results. The graph at left show results of a larger study with an earlier version of the RTS. The graph compares liver iron concentrations (LIC) from the RTS and the existing SQUID susceptometer at St. Anne’s Hospital, Torino, Italy. This comparison was done by our collaborators at the hospital and at the University of Verona. It involved approximately 80 patients with thalassemia, who had varying degrees of iron overload caused by repeated blood transfusions. This work was presented at a recent meeting of the American Society of Hematology.
We estimate that the accuracy achieved with this earlier model was suitable to detect high iron levels that call for increased doses of iron-removing drugs in transfusion-dependent patients with thalassemia. Since this work, we have made a number of improvements to make the liver iron measurements more accurate, so that it can be used for finer adjustments of iron-removing therapy at the lower levels that doctors prefer to maintain in their patients. The Oakland results shown earlier are a first test of this improved system. Ultimately, we hope to measure even the much subtler iron overload that occurs in many millions of patients with liver disease.
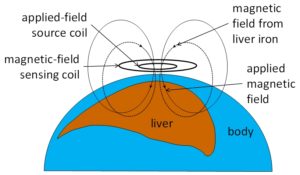
How the liver-iron sensor works. Our room-temperature liver susceptometer senses the magnetization of liver iron in response to an applied magnetic field. As shown in the diagram, we place a sensing unit next to the patient’s thorax, at a point near the middle of the liver. The sensing device produces a magnetic field. That applied magnetic field causes iron in the liver to become slightly magnetized. That magnetization produces its own magnetic field, which is proportional to the liver iron concentration. We measure that magnetic-field response and use it to calculate the liver iron concentration.
A major challenge in this kind of measurement is that the magnetic field produced by the magnetization of liver iron can be 100 million times less intense than the magnetic field that is applied to the patient’s thorax. Our device can be inexpensive because we approach that problem with a strategically designed measurement technique instead of complicated technology. Here are two examples:
One challenge in liver susceptometry is just that the magnetic sensor has to be sensitive enough to pick up the tiny magnetic field produced by liver iron. The SQUID system solves this problem using an ultrasensitive Superconducting QUantum Interference Device. The use of this type of sensor is part of the reason that the SQUID system requires liquid helium. The RTS eliminates the need for sophisticated magnetic sensors by using magnetic fields that oscillate, or reverse direction, several hundred times per second. These rapidly varying magnetic fields are much easier to detect. The RTS produces and detects magnetic fields using coils of ordinary copper wire at room temperature.
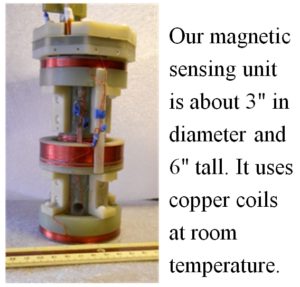 That coil unit, shown in the photograph at left, is about 3″ in diameter and 6″ tall. Its small size means that future versions of this technology can be compact systems that fold up to roll through hospital corridors or break down into packages that fit into the trunk of a car.
That coil unit, shown in the photograph at left, is about 3″ in diameter and 6″ tall. Its small size means that future versions of this technology can be compact systems that fold up to roll through hospital corridors or break down into packages that fit into the trunk of a car.
The second key problem comes from temperature fluctuations that cause parts of the sensing system to expand and contract slightly, minutely changing the distance between the source coil that creates the applied magnetic field and the sensing coil that measures changes in magnetic field. Changes in that distance create unwanted signals that mask the signal from liver iron.
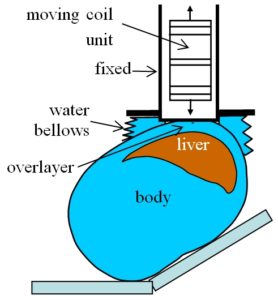
The SQUID solves this problem by using liquid helium to cool the entire sensor system to a temperature of -460° F, at which thermal expansion and contraction are greatly reduced. In contrast, the RTS circumvents the problem by moving the sensing system periodically toward and away from the patient. With this sensor motion, the signal due to liver iron grows and shrinks in a regular way, which is easily distinguishable from the slow, irregular fluctuations caused by temperature change.
This strategy allows us to detect tiny changes in magnetic field, using readily available materials and components: a set of copper coils, a motor to drive the sensor motion, some basic electronics to drive the source coils and measure the signal voltage, and a computer to analyze the results.
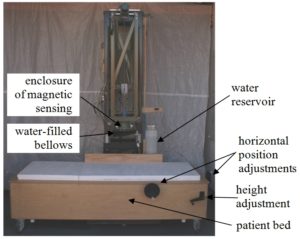
Main components of the RTS. The diagram above shows the main parts of the RTS. The magnetic sensing unit, whose outer enclosure is indicated in the figure, is suspended from a framework that stands on the floor. Below the sensing unit is a patient bed, which can be moved vertically and horizontally to position the patient under the sensor unit. Surrounding the outer enclosure of the sensing unit is a water-filled bellows, which can be expanded to fill the space between the sensing unit and the patient. The system also includes a computer and monitor, data acquisition system, and electronics to supply electric current to the source coils and measure the signal voltage in the sense coils (not shown).
In contrast with the SQUID, which is a fixed installation in its own special room, the RTS can be installed in any room. It can be moved easily on casters, and its total floor footprint is only equivalent to two hospital beds plus a computer workstation.
How liver iron measurements are done. Liver iron measurements with the RTS are very similar to those with the existing SQUID systems. They involve the following steps:
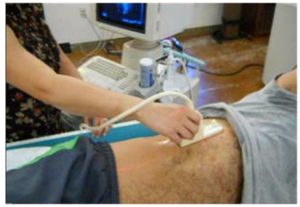
1. Selecting a point over the liver. The subject lies on a horizontal bed with the right side of the thorax facing upward. Ultrasound imaging is used to select a spot on the patient’s skin over the center of the liver, as shown in the figure at left.
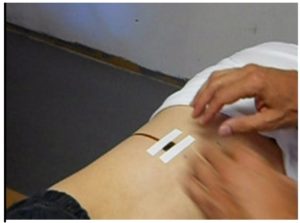
2. Marking the point with a locator loop. Once a suitable spot has been selected, the spot is marked by taping a small “locator loop” to the skin, as shown in the photograph. The locator loop is a set of wire loops approximately 3/4 of an inch in diameter, encased in a flat, flexible piece of plastic. The one we use is identical to the one used with the SQUID.
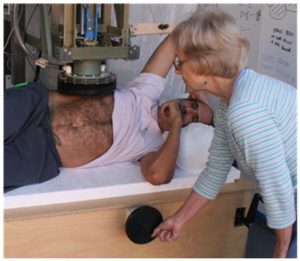
3. Centering the patient under the magnetic sensing system. Once the locator loop has been attached, the patient is centered under the magnetic measurement system, using the locator loops to sense the magnetic field that the system generates. During this step, the patient bed’s height adjustment and horizontal position adjustments are used to bring the top of the patient’s thorax within two or three millimeters of the sensing unit’s bottom enclosure, with the locator loop immediately below the center of the susceptometer’s magnetic sensing system.

4. Filling the water bellows. After centering the patient, the water bellows is expanded to fill the space between the patient and the magnetic sensing unit. The water bellows, also used with the SQUID, helps to remove errors caused by the water-like magnetic-field response of body tissue.
5. Measuring the magnetic-field response. With the patient centered and the water bellows filled, the patient is asked to hold his or her breath for ten seconds, while the magnetic-field response is measured. Typically, nine such measurements are taken on each patient.
The steps above, which are the only ones that require the patient to be present, typically require twenty to thirty minutes altogether. The complete liver iron measurement also involves two other steps, which can be performed in the patient’s absence:

6. Reference measurement on water. To correct for the magnetic-field response of the patient’s body tissue, the liver iron value is determined from the difference between the patient’s response and that of a water-filled space created by expanding the water bellows. Typically, one water reference measurement is made just before positioning the patient under the magnetic sensing system, and another is made just after finishing the magnetic measurements on the patient.
7. Liver iron calculation. The patient’s liver iron concentration is calculated from the difference between the magnetic field response of the patient and that of water. This calculation takes into account the distance from the sensor unit to the patient’s thorax, as determined from the locator loop, the distance from the liver to the outer surface of the body, as determined from ultrasound imaging, and the curved shape of the upper part of the liver, also determined from ultrasound imaging.
Research Collaborations. A number of medical research groups have collaborated with us in studies using our room-temperature liver susceptometer:
Columbia Presbyterian Hospital, New York City: Gary Brittenham, MD. Dr. Brittenham worked with us to do our very first test of the room-temperature susceptometer, comparing our system with SQUID in patients with thalassemia. Those early results are described in a paper in Physiological Measurement. This work was supported by the U.S. National Institute of Diabetes and Digestive and Kidney Disease, contract #s N 43-DK-7-2250 and N44-DK-09-2309.
University of Verona, Verona, Italy: Prof. Alberto Fenzi; Ospedale Ostetrico Ginecologico S. Anna, Torino, Italy: Antonio Piga, MD, Filomena Longo, MD. These two Italian research groups collaborated to compare an early version of our susceptometer with SQUID in 84 patients with thalassemia and other conditions. The results are shown under “Previous results” on this webpage. This work is also described in a recent abstract.
Virginia Mason Medical Center, Seattle, Washington: Dr. Krishnamurthy Kowdley. Dr. Kowdley’s group worked with us in a comparison of susceptometry with liver biopsy in patients with liver disease and other iron overload conditions. This study showed that, to resolve mildly elevated liver iron concentrations in overweight patients, a capability needed for applications in liver disease, better methods would be needed to correct for the magnetic susceptibility of the layer of tissue between the liver and the magnetic sensing unit that is placed next to the skin. Results are described in a paper in Annals of Hepatology. This work was supported by the U.S. National Institute of Diabetes and Digestive and Kidney Diseases, grant #s NIDDK R21 DK072360 and NIDDK K24 DK002957.
Mary Gooley Hemophilia Center & University of Rochester, Rochester New York: Pradyumna Phatak, MD. Working with Dr. Phatak’s group, we tested a new technique for canceling out the interfering signal from the tissue layer between the liver and the susceptometer’s sensor unit, in order to measure liver iron concentrations more accurately in overweight patients. This work was supported by the U.S. National Institute of Diabetes and Digestive and Kidney Diseases, grant # NIDDK 5 R21 RR020211-02.
Center for Sickle Cell Disease, Howard University, Washington, DC: Victor Gordeuk, MD and Patricia Oneal, MD. Dr. Gordeuk and Dr. Oneal worked with us to test another technique for measuring liver iron concentrations more accurately, especially in overweight patients, by correcting for the interfering signal of the tissue between the liver and the sensor unit. Compared with the method tested in Rochester (see above), this method used a simpler, smaller sensor unit that would lend itself better to a compact, easily transportable clinical device. These tests involved evaluating the scatter of the liver iron measurements in healthy adults with normal liver iron concentrations. This work was supported by the U.S. National Center for Minority Health Disparities, grant Number 1R41 MD005715-01.
University of Heidelberg and Salem Medical Center, Heidelberg, Germany: Sebastian Mueller, MD. Using the susceptometer system that was initially tested in collaboration with Howard University Hospital (see above), the Heidelberg group did an extensive series of experiments involving liver iron measurements in patients with liver disease and other conditions. These experiments included a comparison with liver biopsy in 35 patients. The results are described in a paper in the Journal of Hepatology. This work was supported by grants from the Dietmar Hopp Foundation and the German Research Foundation.
The recent paper by the Heidelberg group proposes that the room-temperature susceptometer predominantly detects iron in the hepatocytes, one of the two main types of cells in the liver, rather than the other main cell type, the macrophages. Our own analysis suggests that the data so far could also be explained simply by assuming that, in this study population, most of the liver iron was in the hepatocytes. More research is needed to test the Heidelberg group’s proposal in a statistically strong way.
Comprehensive Thalassemia Center, UCSF Benioff Children’s Hospital Oakland, Oakland, California: Ashutosh Lal, MD and Marcela Weyhmiller, PhD. Oakland is one of the leading clinical and research centers for thalassemias and sickle cell disease in the United States. Dr. Lal and Dr. Weyhmiller worked with us to compare the latest version of the RTS with SQUID in three normal control subjects and twelve transfusion-dependent thalassemia patients with iron overload. These data are presented under “Latest results” on this web page. The experiments are described in a recent abstract.
Results from this small pilot study will guide the design of a larger future study to evaluate the accuracy that can be achieved with the improvements that we have made since the previous SQUID comparison conducted by the groups in Torino and Verona. This work may ultimately lead to the placement of systems at thalassemia clinics in West Bengal, Sri Lanka and other areas where there is an urgent need for iron monitoring.